se valence electrons|Iba pa : Tagatay Mar 23, 2023 With this resistor color code calculator, you'll quickly and easily find out the resistance of your resistor component. Just choose how many bands your resistor has – 4, 5, or 6, select the colors , and in the blink of an eye, you'll get the resistance with tolerance, range, and temperature coefficient value (if you've chosen 6 band resistor .
PH0 · valence electrons for kids
PH1 · valence electrons chart
PH2 · valence electrons calculator
PH3 · se valence electron configuration
PH4 · list of valence electrons for each element
PH5 · how do you find valence electrons
PH6 · find valence electrons on periodic table
PH7 · Iba pa
PH8 · 6 valence electrons least mass
LootedPinay.com delivers best Pinay Porn and Pinay Sex Scandal Video For You, from Kantotflix, katorsex and kayatan Site. Skip to content. Home; Yong sabik lage sa ng bagong client. 1 05:07. Tite ba naman ni tito kinain nya rin. 0 05:02. Tahol ka ng tahol sa laki ng batuta ni dagul. 0 03:37. Gusto ng video pero may mask.
se valence electrons*******Mar 23, 2023 There are two ways to find the number of valence electrons in Selenium (Se). The first is to use the Periodic Table to figure out how many electrons Selenium has in its valence shell. .
Here is a table of element valences. Remember that an element's electron . Valency of Selenium. Selenium can hold multiple valencies in different occasions as per its compound. It may generally have the valency of -2,4 and 6. The electron configuration of this chemical .
Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the .Electron affinity: 194.959 kJ/mol: Oxidation states: −2, −1, +1, +2, +3, +4, +5, +6 (a strongly acidic oxide) Ionization energies: 941 kJ/mol; 2045 kJ/mol; 2973.7 kJ/mol; 4144 kJ/mol; .Red Se, Grey Se, Black Se Se Selenium 34. 78.971 . Group A vertical column in the periodic table. Members of a group typically have similar properties and electron .
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) .
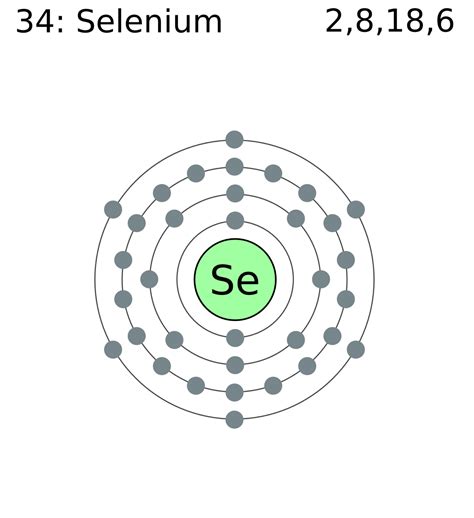
se valence electrons Iba pa Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) .se valence electrons 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding .Selenium is a chemical element of the periodic table with chemical symbol Se and atomic number 34 with an atomic weight of 78.9718 u and is classed as a nonmetal. . Valency electrons : 2,4,6: Bohr model: Electron shell for Selenium, created by Injosoft AB Se. Figure: Shell diagram of Selenium (Se) atom. Orbital Diagram. 1s: 2s: 2p: 3s: 3p: 3d . This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.Iba pa Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s . As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .
Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Valency of Selenium. Selenium can hold multiple valencies in different occasions as per its compound. It may generally have the valency of -2,4 and 6. The electron configuration of this chemical element is 2-8-18-6. It clearly has the 6 electrons in its outer shell to have variable valency. Filed Under: Period Table. sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .
Generally, valence electrons can participate in the formation of chemical bonding, but core electrons cannot. While core electrons are not involved in bonding, they influence the chemical reactivity of an atom. The electron configuration of a oxygen atom is. O: 1s22s22p4 (1.9B.1) (1.9B.1) O: 1 s 2 2 s 2 2 p 4. which may be shorted.

In the above electron configuration, the highest energy level (4) is marked with green color. The 4 th energy level contains 4s and 4p subshells. There are 2 electrons in the 4s subshell and 4 electrons in . Selenium consists of 34 electrons distribution in its 4 orbits. So electronic configuration of selenium define as: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 3d10 4p 4. Or. The electronic configuration can also be represented with the help of a full electron distribution element that is Argon/ Ar. In this case it is written as [Ar] 3d 10 4s 2 4p 4.
Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron. For example, alkali metals, which all have a valency of .Red Se, Grey Se, Black Se Se Selenium 34. 78.971 . Group A vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. .
Except for helium, they all have eight valence electrons. Chemists have concluded that atoms are especially stable if they have eight electrons in their outermost shell. This useful rule of thumb is called the octet rule, . Selenium is more likely to gain two electrons. It will become Se 2 . Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .
Find your ideal job at Jobstreet with 98 Fresh Graduate Remote jobs found in Philippines. View all our Fresh Graduate Remote vacancies now with new jobs added daily!
se valence electrons|Iba pa